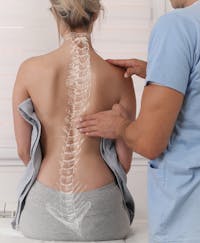
When it comes to Adolescent Idiopathic Scoliosis surgery, New Jersey spine surgeon Dr. Joshua Rovner has the comprehensive experience patients trust. As one of the states leading destinations for spine surgery in NJ, Adolescent Idiopathic Scoliosis is a spinal condition that Dr. Rovner and his advanced surgical team treats.
Treatment of Adolescent Idiopathic Scoliosis
To achieve the best success in managing a child’s AIS he or she is going to need three things including early detection, Progressive Spine and Orthopaedics state of the art treatment, and a positive outlook. Most children will see results with the effective use of braces.
However, in rare cases, spine surgery might be needed to help correct more severe cases of scoliosis. If needed, Dr. Joshua Rovner will discuss every option with you. The vast majority of these spinal procedures are minimally invasive. The last thing a child will need for a healthy recovery is a positive outlook on their condition.
With an ailment such as scoliosis it is not uncommon for adolescents to suffer from body image and confidence issues. At Progressive Spine & Orthopaedics we treat each patient with respect in an open and caring environment. To us, every patient is treated as an individual.